Which Best Describes the Mass of Protons Neutrons and Electrons
17 What are the roles of protons neutrons and electrons in an atom. Which statement best describes the electrons in this illustration.
Electrons Have Some Mass But When We Count The Atomic Mass It Is Equal To The Mass Of The Proton And Neutron Then Why Don T We Count The Mass Of E In
If the charge is negative electrons are in excess.

. Which of the following best describes an isotope. Neutrons and electrons are in the nucleus with protons orbiting the nucleus. Neutrons have a much larger mass than protons and electrons.
An ion has an unequal number of protons and electrons. If the charge is positive there are more protons than electrons. Number of protons 11.
The atomic mass of an atom is the sum of the mass of its protons neutrons and electrons. To find the number of neutrons subtract the number of protons from the atomic mass. 10 What is the value of a proton.
Protons and neutrons are in the center of the atom making up the nucleus. 7 Which statement explains why most of the mass of an atom is contained in the small nucleus. 13 How many neutrons are in the nucleus of the atom.
11 How many coulombs Does a proton have. The atomic mass of an atom is the same as the atoms atomic number. If an element has 15 protons and 16 neutrons and 15 electrons what is the atomic mass of the element.
14 What is the mass of electron proton and neutron. Neutral subatomic particle located in the nucleus. Number of protons plus the number of neutrons.
Its mass is lowered but it is still the same element. As we know that the masses of proton electron and neutron is given as. A Structurally variant atoms which always have a mass number of 1.
12 Why are protons and electrons equal. 8 Which statement best explains why the overall charge on an atom is zero quizlet. 6 How do gluons hold quarks together.
1 What Holds Neutrons And Protons Together. 5 Which statement best describes the structure of an atom. Question 4 2 points Which statement below best describes the structure of an atom.
The atomic mass of an atom is the sum of the mass of its protons and electrons. 3 rows The mass of a neutron is slightly greater than the mass of a proton which is 1 atomic mass. Electrons have a negative charge.
Negatively charged subatomic particle that orbits the nucleus. 3 How does a proton and neutron bind. 6 Why is it important to know the structure of an atom.
Electrons are positively charged particles in the nucleus. 7 What forces hold the atom together. Electrons have a much larger mass than protons and neutrons.
Atoms are made of extremely tiny particles called protons neutrons and electrons. What happens if an atom loses a neutron. The atomic mass of an atom is the sum of the mass of its neutrons and electrons.
A12 protons 12 neutrons and 12 electrons B11 protons 11 neutrons and. 18 Which of the following statements best describes the role of neutrons in the nucleus. The atomic mass of an atom is the sum of the mass of its protons neutrons and electrons.
15 Why do neutrons and protons attract. What is the atomic number of an oxygen atom with 8 protons and 10 neutrons in its nucleus. And also we can see that protons and neutrons are of similar mass range.
16 How are neutrons added to an atom. So correct answer would be. 4 Why are protons and electrons attracted to each other.
112 rows Lithium has 3 protons 4 neutrons and 3 electrons. 5 Which force is most responsible for binding together an atoms protons and neutrons. 7 Are protons and electrons equal.
A critical mass will detonate when it is separated into subcritical pieces. Protons have a much larger charge than neutrons and electrons. An atom has 3 protons 4 neutrons and 3 electrons.
2 Why would protons and neutrons hold together. The atomic mass of an atom is the sum of the mass of its protons neutrons and electrons. 8 How many times proton is heavier than electron.
Electrons are negatively charged particles in the nucleus. The charge on the proton and electron are exactly the same size but opposite. Protons have a positive charge.
An electron has a negative charge and is located outside the nucleus an ion that consists of 7 protons 9 neutrons and 10 electrons has a net charge of. Number of neutrons mass number - atomic. In more microscopic way if we see them then neutrons are slightly heavier than the proton.
4 What defines the mass of an atom. Simply subtract the number of protons the atomic number from the mass number to find the remaining neutrons. Number of electrons 11.
Which statement describes atomic mass. 1Which of the following best describes an atom. 14 What holds protons and neutrons together in the nucleus.
Which best describes the mass of protons neutrons and electrons. 13 What is the mass of an electron answer. Which statement best describes the electrons in this illustration.
So here we can say that electron is of least mass among all three. 9 What is the value of mass on proton. Beryllium has 4 protons 5 neutrons and 4 electrons.
16 31 15 30 none of the above. B Structurally variant atoms which have the same number of neutrons and protons but differ in the number of electrons they contain. Use the periodic table to determine which atom would have similar chemical properties to this atom.
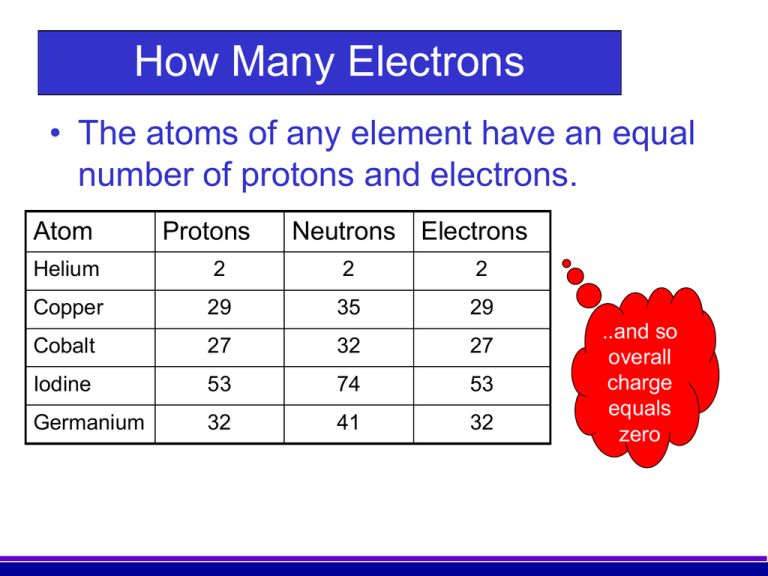
Neutrons and protons have a much larger mass than electrons. The atomic mass of every atom of an element such as carbon is exactly the same. The of electrons e- is the same as the of protons if the atom has no charge.
Which of the following statements best describes a critical mass. You can find the number of neutrons if you know the isotope of the atom. Electrons surround the nucleus.
Boron has 5 protons 6 neutrons and 5 electrons.

The Structure Of The Atom Boundless Chemistry

Image Result For Aluminum Atom Model Modelos Atomicos Atomo Ciencias Naturais
What Is The Relation Between The Mass Of An Electron And A Proton Quora
0 Response to "Which Best Describes the Mass of Protons Neutrons and Electrons"
Post a Comment